SERIOUS INFECTIONS
Patients treated with ENBREL are at increased risk for developing serious infections that may lead to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids or were predisposed to infection because of their underlying disease. ENBREL should not be initiated in the presence of sepsis, active infections, or allergy to ENBREL or its components. ENBREL should be discontinued if a patient develops a serious infection or sepsis. Reported infections include: 1) Active tuberculosis (TB), including reactivation of latent TB. Patients with TB have frequently presented with disseminated or extrapulmonary disease. Test patients for latent TB before ENBREL use and periodically during therapy. Initiate treatment for latent infection prior to ENBREL use, 2) Invasive fungal infections, including histoplasmosis, coccidioidomycosis, candidiasis, aspergillosis, blastomycosis, and pneumocystosis. Patients with histoplasmosis or other invasive fungal infections may present with disseminated, rather than localized, disease. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. Consider empiric antifungal therapy in patients at risk for invasive fungal infections who develop severe systemic illness, and 3) Bacterial, viral, and other infections due to opportunistic pathogens, including Legionella and Listeria.
The risks and benefits of treatment with ENBREL should be carefully considered prior to initiating therapy in patients 1) with chronic or recurrent infection, 2) who have been exposed to TB, 3) who have resided or traveled in areas of endemic TB or endemic mycoses, or 4) with underlying conditions that may predispose them to infections such as advanced or poorly controlled diabetes. Monitor patients closely for the development of signs and symptoms of infection during and after treatment with ENBREL, including the possible development of TB in patients who tested negative for latent TB prior to initiating therapy.
MALIGNANCIES
Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with tumor necrosis factor (TNF) blockers, including ENBREL.
In adult clinical trials of all TNF blockers, more cases of lymphoma were seen compared to control patients. The risk of lymphoma may be up to several-fold higher in RA patients. The role of TNF blocker therapy in the development of malignancies is unknown.
Cases of acute and chronic leukemia have been reported in association with postmarketing TNF blocker use in RA and other indications. The risk of leukemia may be higher in patients with RA (approximately 2-fold) than the general population.
Melanoma and non-melanoma skin cancer (NMSC) have been reported in patients treated with TNF blockers, including ENBREL. Periodic skin examinations should be considered for all patients at increased risk for skin cancer.
Pediatric Patients
In patients who initiated therapy at ≤18 years of age, approximately half of the reported malignancies were lymphomas (Hodgkin's and non-Hodgkin's lymphoma). Other cases included rare malignancies usually associated with immunosuppression and malignancies that are not usually observed in children and adolescents. Most of the patients were receiving concomitant immunosuppressants.
NEUROLOGIC REACTIONS
Treatment with TNF-blocking agents, including ENBREL, has been associated with rare (<0.1%) cases of new onset or exacerbation of central nervous system demyelinating disorders, some presenting with mental status changes and some associated with permanent disability, and with peripheral nervous system demyelinating disorders. Cases of transverse myelitis, optic neuritis, multiple sclerosis, Guillain-Barré syndromes, other peripheral demyelinating neuropathies, and new onset or exacerbation of seizure disorders have been reported in postmarketing experience with ENBREL therapy. Prescribers should exercise caution in considering the use of ENBREL in patients with preexisting or recent-onset central or peripheral nervous system demyelinating disorders.
NEW ONSET OR WORSENING OF HEART FAILURE
Cases of worsening congestive heart failure (CHF) and, rarely, new-onset cases have been reported in patients taking ENBREL. Caution should be used when using ENBREL in patients with CHF. These patients should be carefully monitored.
HEMATOLOGIC REACTIONS
Rare cases of pancytopenia, including aplastic anemia, some fatal, have been reported. The causal relationship to ENBREL therapy remains unclear. Exercise caution when considering ENBREL in patients who have a previous history of significant hematologic abnormalities. Advise patients to seek immediate medical attention if they develop signs or symptoms of blood dyscrasias or infection. Consider discontinuing ENBREL if significant hematologic abnormalities are confirmed.
HEPATITIS B REACTIVATION
Reactivation of hepatitis B has been reported in patients who were previously infected with hepatitis B virus (HBV) and received concomitant TNF-blocking agents, including ENBREL. Most reports occurred in patients also taking immunosuppressive agents, which may contribute to hepatitis B reactivation. Exercise caution when considering ENBREL in these patients.
ALLERGIC REACTIONS
Allergic reactions associated with administration of ENBREL during clinical trials have been reported in <2% of patients. If an anaphylactic reaction or other serious allergic reaction occurs, discontinue administration of ENBREL and initiate appropriate therapy immediately.
IMMUNIZATIONS
Avoid concurrent administration of live vaccines with ENBREL. Patients, if possible, should be brought up to date with all immunizations prior to initiating ENBREL. In patients with exposure to varicella virus, temporarily discontinue ENBREL and consider prophylactic treatment with Varicella Zoster Immune Globulin.
AUTOIMMUNITY
Autoantibodies may develop with ENBREL, and rarely lupus-like syndrome or autoimmune hepatitis may occur. These may resolve upon withdrawal of ENBREL. Stop ENBREL if findings suggestive of lupus-like syndrome or autoimmune hepatitis develop and evaluate the patient.
NOT RECOMMENDED FOR GRANULOMATOSIS WITH POLYANGIITIS PATIENTS ON IMMUNOSUPPRESSANTS
The use of ENBREL in patients with granulomatosis with polyangiitis receiving immunosuppressive agents (eg, cyclophosphamide) is not recommended.
INCREASED MORTALITY IN PATIENTS WITH MODERATE TO SEVERE ALCOHOLIC HEPATITIS
Based on a study of patients treated for alcoholic hepatitis, exercise caution when using ENBREL in patients with moderate to severe alcoholic hepatitis.
ADVERSE REACTIONS
The most commonly reported adverse reactions in RA clinical trials were injection site reaction and infection. In clinical trials of all other adult indications, adverse reactions were similar to those reported in RA clinical trials.
In general, the adverse reactions in pediatric patients were similar in frequency and type as those seen in adult patients. The types of infections reported in pediatric patients were generally mild and consistent with those commonly seen in the general pediatric population.
DRUG INTERACTIONS
The use of ENBREL in patients receiving concurrent cyclophosphamide therapy is not recommended. The risk of serious infection may increase with concomitant use of abatacept therapy. Concurrent therapy with ENBREL and anakinra is not recommended. Hypoglycemia has been reported following initiation of ENBREL therapy in patients receiving medication for diabetes, necessitating a reduction in anti-diabetic medication in some of these patients.
Please see Prescribing Information and Medication Guide.
INDICATIONS
ENBREL is indicated for reducing signs and symptoms, inducing major clinical response, inhibiting the progression of structural damage, and improving physical function in patients with moderately to severely active rheumatoid arthritis. ENBREL can be initiated in combination with methotrexate (MTX) or used alone.
ENBREL is indicated for reducing signs and symptoms of moderately to severely active polyarticular juvenile idiopathic arthritis in patients 2 years of age and older.
ENBREL is indicated for reducing signs and symptoms, inhibiting the progression of structural damage of active arthritis, and improving physical function in adult patients with psoriatic arthritis. ENBREL can be used with or without MTX.
ENBREL is indicated for reducing signs and symptoms in patients with active ankylosing spondylitis.
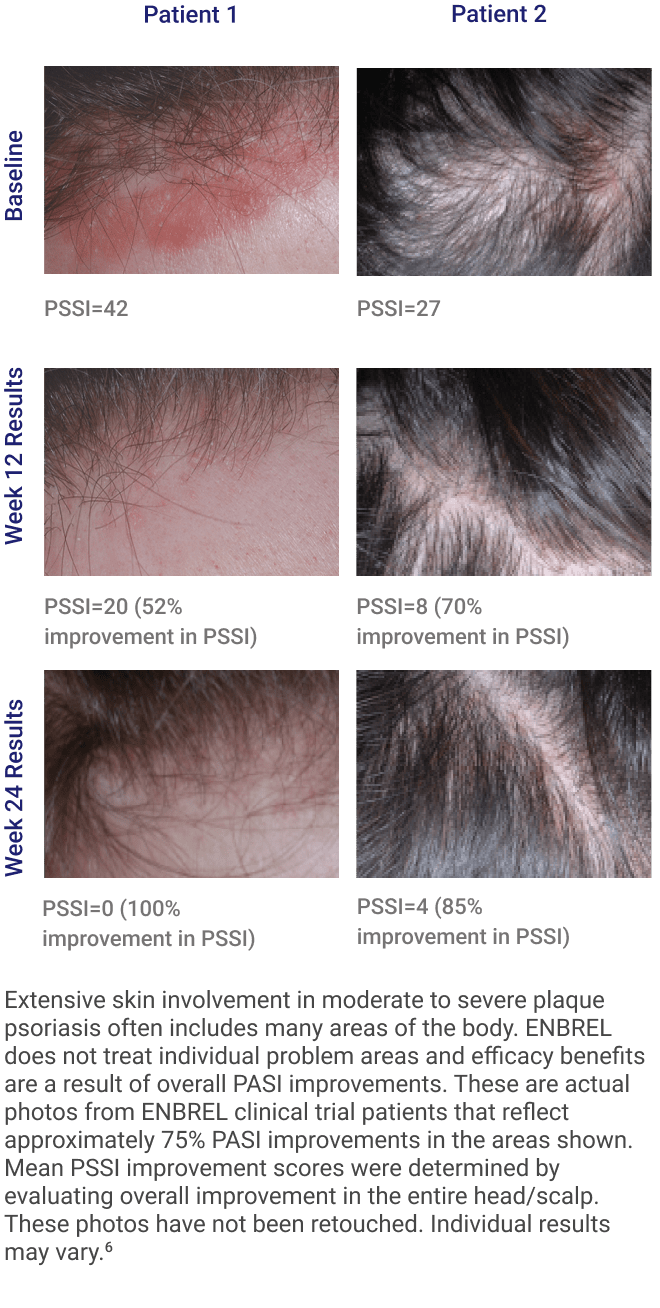
ENBREL is indicated for the treatment of patients 4 years or older with chronic moderate to severe plaque psoriasis (PsO) who are candidates for systemic therapy or phototherapy.
ENBREL is indicated for the treatment of active juvenile psoriatic arthritis in pediatric patients 2 years of age and older.